Chelating & Reducing Agents
Biochemicals
Chelating & Reducing Agents
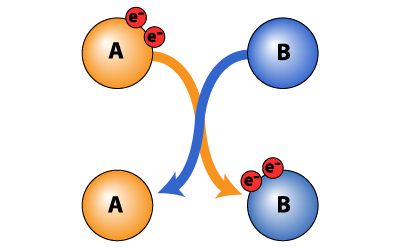
Reducing agents, also known as reducers or reductants, are essential for studying protein in biochemistry1 and are used in various techniques in proteomics, such as protein denaturation and solubilization. They are especially useful in stabilizing free sulfhydryl groups and reducing disulfide bonds of proteins and peptides2. These reducers donate electrons in a redox chemical reaction (Figure 1). Reagents, such as dithiothreitol (DTT) also called Cleland’s reagent, dithioerythritol (DTE), tris(2-carboxyethyl)phosphine (TCEP), β-mercaptoethanol (BME), sodium dithionate, and nitrilotriacetic acid (NTA) are used widely as noble-reducing agents.
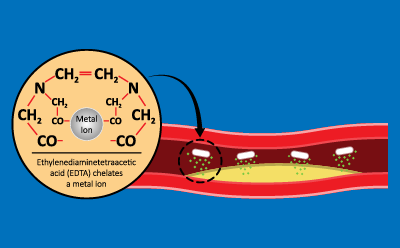
Chelators, or chelating agents, remove heavy metals3 by binding to metal ions to form two or more bonds with their ring-like structure to build a water-soluble, stable structure. Common chelating agents include ethylenediaminetetraacetic acid (EDTA), ethylene glycol tetraacetic acid (EGTA) and ethylenediamine (Figure 2). Research has demonstrated that using chelating reagents, in combination with reducing reagents, supports in the removal of cationic heavy metals as well as anionic contaminants, such as arsenic4.
Choose from our comprehensive range of reducing and chelating agents, available in various high purities and forms, such as liquid, solution, crystalline solid, and powder, for your scientific advancement.


