Click Chemistry Reagents
Chemical Biology
Click Chemistry Reagents
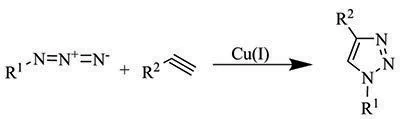
Our extensive portfolio of click chemistry reagents offers a variety of azides, alkynes, catalysts, and ligands to help accelerate your research in the exciting arena of “click” chemistry. Click chemistry is a term coined by Barry Sharpless to describe chemical reactions that are modular, efficient, wide in scope, provide very high yields, and generate only inoffensive byproducts. The most well-known example of a “click” reaction is the Copper(I)-catalyzed Azide-Alkyne 1,3-dipolar Cycloaddition (CuAAC), which yields a 1,4-disubstituted five-membered 1,2,3-triazole ring.
This reaction between azides and alkynes offers high yields and involves functionalities that can be introduced relatively easily in a variety of molecules such as synthetic polymers, fluorophores, small molecules or into specific locations in biomolecules. An advantage of this reaction for biological purposes is that the azide and alkyne functional groups are largely inert, or biorthogonal, towards biological molecules and aqueous environments. “Click” chemistry continues to gain popularity and is used in a variety of research fields with significant contributions to the fields of chemical biology, polymer chemistry, bioconjugation, and drug discovery.


