Cross-Coupling Catalysts
Catalysts
Cross-Coupling Catalysts
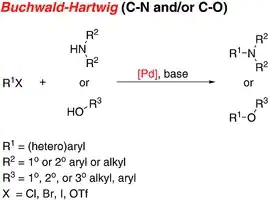
Cross-coupling is a cornerstone set of reactions for the formation of carbon–carbon and carbon-heteroatom bonds. Numerous research groups have developed new metal complexes and ligands, expanding the scope of these transformations to give access to more complex molecules. Among these important reactions, several stand out, such as Suzuki–Miyaura, Negishi, Heck, Kumada, Stille, Sonogashira and Buchwald Hartwig amination reactions. Furthermore, scientists have developed new complexes that are able to catalyze these reactions, with high yields and low catalyst loadings. Several of the technologies developed enable these reactions to be carried out under mild reaction conditions, with high activity and high turnover numbers. Several of these catalysts have transitioned into industry successfully, catalyzing cross-coupling reactions on a ton scale.
We are dedicated to supporting all of your needs with a comprehensive cross-coupling catalysts portfolio:
MPHOS: A TUNABLE LIGAND SCAFFOLD
MPhos is a new class of unsymmetrical bis-phosphino ferrocene ligands that can be used in a variety of cross-coupling reactions. The ligand scaffold includes a bulky di(1-adamantyl)phosphino motif as well as a tunable second phosphine. Demonstrated applications include many types of Csp2-Csp3 couplings (i.e. Murahashi−Feringa (Li), Kumada−Corriu (Mg), Negishi (Zn) and Suzuki−Miyaura (B)) with broad substrate scope including many “drug-like” molecules. Learn more here.
NICKEL CATALYSTS
We are pleased to offer an extensive and high-purity selection of Ni catalysts. These nickel catalysts span a range of oxidation states: Nickel (0), nickel (II), nickel (III) and nickel (IV). The types of Ni catalysts available for immediate purchase are aluminum nickel (Al Ni) alloys, ammonium nickel hydrates, Ni COD, Ni halides (chlorides, bromides, fluorides and iodides), Ni cyclopentadienyls, nickel metal, nickel acacs, and Raney Nickel catalysts.
PALLADIUM CATALYSTS
Palladium catalysts are extremely versatile due to the ability to fine-tune the reaction conditions (temperature, solvents, ligands, bases, and other additives). Furthermore, palladium catalysts have a very high tolerance of various functional groups and often provide excellent stereo- and regiospecificity, which avoids the need for protecting groups. We offer an extensive portfolio of homogeneous and heterogeneous palladium catalysts.


