Organozinc Reagents
Chemical Synthesis
Organozinc Reagents
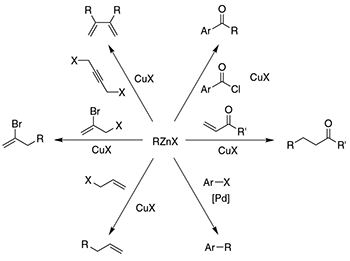
Organozinc reagents, first prepared by Frankland in 1848, are used extensively in organic synthesis as powerful C–C bond-forming tools, participating in Cu(I)-promoted reactions (developed at Rieke Metals, Inc.) or in Pd-catalyzed cross-coupling reactions (i.e. the Negishi coupling), as well as Michael additions, and electrophilic amination reactions. Interestingly, the choice of preparative route to organozinc reagents of the type RZnX (R=alkyl, aryl; X=halide) plays an important role in the reactivity and stability of these compounds.
We offer a comprehensive range of organozinc compounds, including Rieke® Organozinc Reagents that are stable as solutions in tetrahydrofuran, Rieke® Zinc, as well as numerous pre-formed Rieke® organozinc reagents. Prior to the discovery of Rieke® Zinc, it was not possible to react alkyl, aryl, and vinyl bromides or chlorides directly with zinc metal. The preparation of these reagents was only possible by a metathesis reaction of a zinc halide with an organolithium or Grignard reagent. Unfortunately, this approach was of limited utility since the process was not compatible with many types of functional groups. Rieke® Zinc, on the other hand, will react directly with the bromides or chlorides and will tolerate a variety of sensitive groups such as nitriles, esters, amides, ethers, sulfides, and ketones to give functionalized organozinc reagents.
Since many organozinc reagents are pyrophoric, we provide top-quality packaging in Sure/Seal™ bottles to prolong the lifetime of these reagents. A growing list of organometallic reagents are also being transitioned into exclusive 25 mL Sure/Seal packaging to reduce waste and decomposition of more unstable organometallic reagents, by requiring fewer needle entries to consume the reagent volume.
Face your cross-coupling challenges with our reliable range of organozinc reagents to keep your focus on creating breakthroughs.
Chemical Synthesis
- Boronic Acids & Derivatives
- C-C Bond Forming Reagents
- Chiral Auxiliaries
- Coupling Reagents & Nucleosides
- Grignard Reagents
- Halogenation Reagents
- Organoaluminum Reagents
- Organolithium Reagents
- Organosilicon Reagents
- Organotin Reagents
- Organozinc Reagents
- Oxidation Reagents
- Protection/Deprotection Reagents
- Reducing Agents


