Oxidation Reagents
Chemical Synthesis
Oxidation Reagents
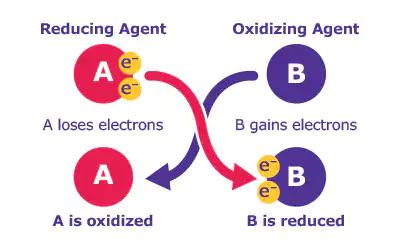
Oxidation-reduction reactions are some of the most common transformations encountered in organic synthesis and are powerful tools for creating novel products. These reactions have manufacturing relevance in small molecule research. No matter what your oxidation-reduction reaction, we have the corresponding oxidation reagents to keep your work flowing.
Selected highlights are:
BAEYER–VILLIGER OXIDATION
The Baeyer–Villiger (BV) oxidation method is the synthetic reaction that oxidizes a ketone to an ester or a cyclic ketone to a lactone. Modifications in 2004 by Brink, Arends, and Sheldon to the BV reaction have made it more sustainable by using hydrogen peroxide as the oxidant.
DESS–MARTIN OXIDATION
Dess–Martin oxidation synthesizes aldehydes or ketones using Dess–Martin periodinane (DMP) as the oxidizing reagent. Due to the mild reaction conditions, it is one of the more preferable oxidation reactions.
Chemical Synthesis
- Boronic Acids & Derivatives
- C-C Bond Forming Reagents
- Chiral Auxiliaries
- Coupling Reagents & Nucleosides
- Grignard Reagents
- Halogenation Reagents
- Organoaluminum Reagents
- Organolithium Reagents
- Organosilicon Reagents
- Organotin Reagents
- Organozinc Reagents
- Oxidation Reagents
- Protection/Deprotection Reagents
- Reducing Agents

