Mass Spectrometry (MS)
Analytical Chemistry
Mass Spectrometry (MS)
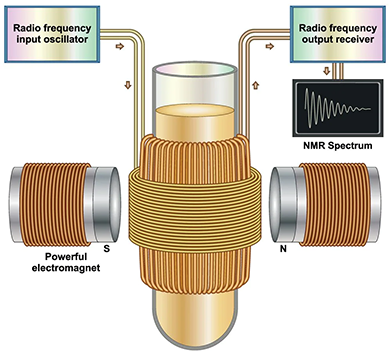
Nuclear Magnetic Resonance (NMR) spectroscopy is an analytical technique used to determine the molecular structure and chemical composition of a sample. It works by analyzing the interaction of spinning nuclei in a strong magnetic field. In NMR spectroscopy, a stationary external magnetic field causes certain nuclei in a molecule to absorb selective radiofrequencies. The energy absorbed induces a transition in nuclear spins, which is observed on an NMR spectrum.
APPLICATIONS OF NMR SPECTROSCOPY
NMR spectroscopy is a non-destructive and non-invasive technique that is used to determine molecular structure and dynamics. The applications of NMR are diverse and include the following research areas and industries:
- In biology, NMR is applied to study macromolecules, such as proteins, lipids and nucleic acids. 13C, 1H, 15N, 31P, 23Na, and 19F are the most biologically relevant NMR-active nuclei, used to understand biochemical pathways involved in amino acid, lipid and carbohydrate metabolism.
- In chemistry, it’s widely used for both qualitative and quantitative analysis to monitor reactions, identify structures and assess purity.
- In polymer science, to analyze monomer ratio, molecular weight, tacticity, sequencing, chain length and branching, and to determine end groups.
- In the pharma industry, to determine the purity and quantity of active ingredients, excipients and impurities in pharmaceutical products
- In the petroleum industry, to assess hydrocarbons of raw petroleum and its products.
- In medicine, magnetic resonance imaging (MRI) is an application of NMR used for soft tissue analysis to identify the damaged or diseased tissues.


